Abstract
Context: Somatic gene mutations contribute to the heterogeneous prognosis of CMML. The hypomethylating agents (HMA) azacitidine (AZA) and decitabine (DAC) are active in CMML. The specific prognostic value of recurrent gene mutations in CMML treated with HMA has never been reported.
Methods: We analyzed a multi-institution retrospective cohort of 183 CMML patients (pts) treated with either AZA or DAC with information on recurrent gene mutations.
Results: CMML pts with a median age of 72 years (WHO 2016: CMML-0/1/2 in 37%, 27% and 36% respectively [resp]) were treated with AZA (n=76) or DAC (n=107) for a median of 7 cycles (InterQuartile Range [IQR]: 4 - 15). Median WBC was 15.1 x109/L, cytogenetic risk according to CPSS (Such et al, Haematologica 2011) was low, int and high in 71%, 12% and 17% of pts, resp. CPSS risk (Such et al, Blood 2013) was low, int-1, int-2 and high in 14%, 23%, 49% and 14%, resp. GFM risk (Itzykson et al, JCO 2013) was low, int and high in 33%, 35% and 32%, resp. The Overall Response Rate (ORR) to HMA was 54%, including 17% of complete responses (CR), without significant differences between AZA and DAC. With a median follow-up of 36.7 months, median overall (OS) and AML-free (AMLFS) survival were 24.4 and 19.2 months, resp. Nineteen (10%) pts were transplanted after HMA and censoring at transplant did not affect our results. Compared to AZA pts, DAC pts had higher WBC (p=0.01) and non-significant trends towards lower platelet count (p=0.07) and poorer cytogenetic risk (p=0.08), with a non-significant trend to a poorer OS (p=0.11). Propensity matching on previous treatment with chemotherapy, WBC, platelets count, peripheral blasts count, cytogenetic risk and ASXL1 status at HMA onset confirmed the lack of OS benefit with AZA in the 144 patients (79%) that could be successfully matched (Hazard Ratio [HR]=1.31, p=0.4). All further analyses were nevertheless stratified on HMA.
Molecular data was obtained from Sanger sequencing (n=74) or NGS (n=109). The molecular spectrum of our cohort was comparable to previous CMML reports and dominated by frequent mutations in SRSF2 (46%), ASXL1 (45%) and TET2 (39%). Our analysis focused on genes analyzed in ≥90% of patients and found mutated in ≥10% (SRSF2, ASXL1, TET2, NRAS, RUNX1 and CBL). Mutation frequencies in these genes did not differ between AZA and DAC pts, nor between Sanger and NGS.
In univariate analysis, a lower ORR was only seen in patients with ASXL1 mutations (ASXL1mut 44% vs ASXL1wt 62%, p=0.02) while only TET2 mutations predicted a higher CR rate (TET2mut 22% vs TET2wt 11%, p=0.02). Multivariate linear regressions confirmed that these differences were independent from clinical parameters.
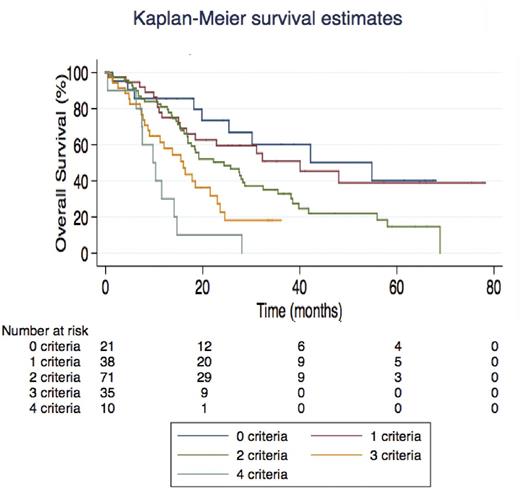
In univariate analysis, mutations in RUNX1 (13% of pts, HR=2.05, p=0.006) and to a lesser extent ASXL1 (45% of pts, HR=1.40, p=0.07) and CBL (12% of pts, HR=1.63, p=0.08) were associated with shorter OS. Similar results were found for AMLFS. There was no significant interaction between TET2 and ASXL1 or SRSF2 status in terms of ORR or OS. Clinical variables associated with poorer OS included splenomegaly (p=0.045), WBC > 13 x 109/L (p=0.003), platelets < 100 x 109/L (p=0.045), peripheral immature myeloid cells (IMC) > 2% (p=0.02), peripheral blasts > 1% (p=0.02) and high-risk karyotype (p=0.047). A multivariate Cox model retained RUNX1 mutations (p=0.007), splenomegaly (p=0.049), WBC > 13 x 109/L (p=0.005), platelets < 100 x 109/L (p=0.046) and high-risk karyotype (p=0.04) as independent predictors of OS. We could therefore build a CMML-HMA model where patients with none, 1, 2, 3 or 4 of these five criteria were found to have distinct median OS ranging from 54.8 to 9.8 months (Figure). This model was internally validated by 5-fold cross validation. Though CPSS (p=0.001) and GFM risk scores both predicted survival of HMA-treated CMML (p≤0.001), our proposed CMML-HMA index proved superior to both risk scores (both likelihood ratio tests: p< 0.05) in our cohort. CPSS-mol (Elena et al, Blood 2016) could not be analyzed due to missing data on SETBP1 status.
Conclusion: Contrary to previous results with smaller patient numbers (Braun et al Blood 2011), treatment with HMA seems to abrogate only partially the poor prognosis of ASXL1 mutations in CMML. RUNX1 mutations confer a poor prognosis in CMML treated with HMA and can be integrated with clinical and hematological parameters to improve prognostication of CMML pts treated with HMA, when compared with "classical" CMML risk scores.
Sallman: Celgene: Research Funding. Coombs: Pharmacyclics: Honoraria; H3 Biomedicine: Honoraria. Renneville: CELGENE: Research Funding. Platzbecker: Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Acceleron: Consultancy, Honoraria, Research Funding. Padron: Incyte: Honoraria, Research Funding. Santini: Celgene: Honoraria, Research Funding; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy; Novartis: Honoraria. Fenaux: Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Itzykson: Janssen: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal